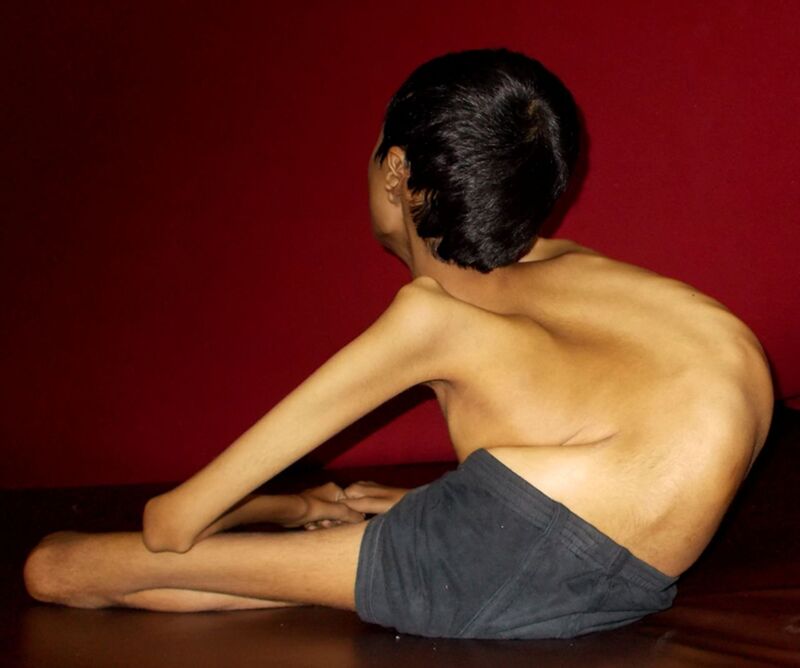
History: A 14 year-old boy presented with progressive proximal weakness of the lower limbs starting at 4 years of age followed by involvement of the upper limbs. He is the product of a consanguineous marriage. Clinically, he had flaccid quadriparesis with wasting and contractures without any sensory or neurological involvement. His weakness worsened leading to an inability to walk without support by the age of 8 and total wheelchair dependence by the age of 11. He was frequently admitted to hospital with chest infections. The patient’s creatine kinase was 2600 IU/L (normal 50–150 IU/L) and muscle biopsy from left quadriceps showed rounded small muscle fibres with evidence of degeneration and an absence of dystrophin protein. What’s the diagnosis?
Answer: Duchenne muscular dystrophy is a X-linked recessive genetic disorder.
Cause: Gene mutation of dystrophin protein.
Signs and symptoms: Muscle weakness, scoliosis, Respiratory Infections, Gowers’s sign.
Diagnosis: Creatine kinase (CPK-MM), DNA test, Muscle biopsy, Prenatal tests.
Treatment:
- Corticosteroids such as prednisolone and deflazacort lead to short-term improvements in muscle strength and function up to 2 years.
- The medication eteplirsen, a Morpholino antisense oligo for the treatment of mutations amenable to dystrophin exon 51 skipping.
- The medication ataluren (Translarna) is approved for use in the European Union.
- The antisense oligonucleotide golodirsen (Vyondys 53) was approved for medical use in the United States in 2019, for the treatment of cases that can benefit from skipping exon 53 of the dystrophin transcript.
- The Morpholino antisense oligonucleotide viltolarsen (Viltepso) was approved for medical use in the United States in August 2020, for the treatment of Duchenne muscular dystrophy (DMD) in people who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping.
- Casimersen (Amondys 45) was approved for medical use in the United States in February 2021, and it is the first FDA-approved targeted treatment for people who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping.