In 2018, Meyers, Weingart and Smith introduced us to the concept of Occlusion Myocardial Infarction (OMI). The term STEMI equivalent is already in our vocabulary, but it actually refers to patients with clinical and ECG features concerning for acute coronary occlusion who would benefit from immediate PCI.
Definition of OMI?
An ongoing ischemia resulting in irreversible infarction caused by complete or near-complete occlusion of a culprit epicardial coronary artery, with inadequate collateral circulation, thus necessitating immediate reperfusion.
Definition of NOMI?
No occlusion, or sufficient collateral circulation to avoid active infarction.
What’s wrong with the STEMI label?
Patients with acute occlusion not meeting STEMI criteria may be an underserved, underidentified subgroup of ACS patients who would benefit from emergent intervention, whereby classification of AMI by occlusion vs. no occlusion may be more appropriate than classification by ST elevation on the ECG. Meyers et al 2020
Example: A 70 year old female presents to ED with atypical chest pain. ECG done.
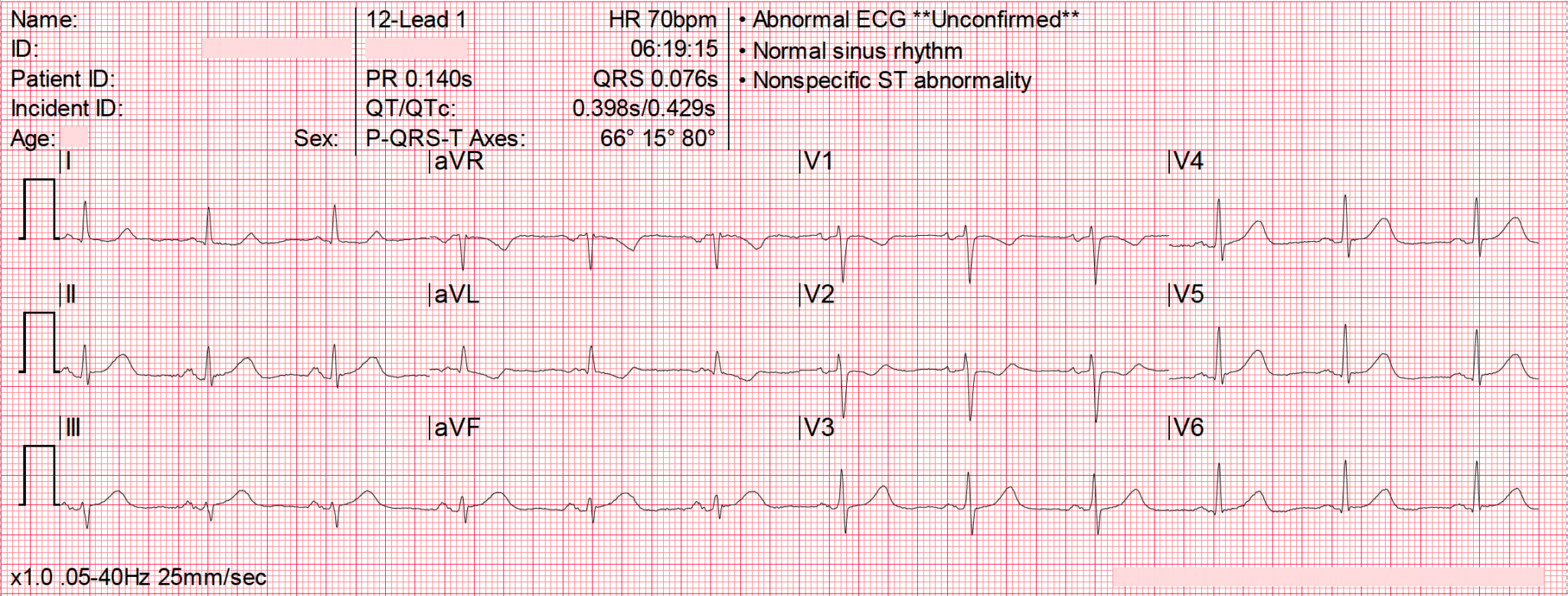
Explanation:
This ECG may appear normal to most of us, but it actually indicates an OMI. This ECG from @tbouthillet shows small hyperacute T waves in inferior leads concerning for early inferior OMI, demonstrated best by their size relative to the preceding QRS Complex.
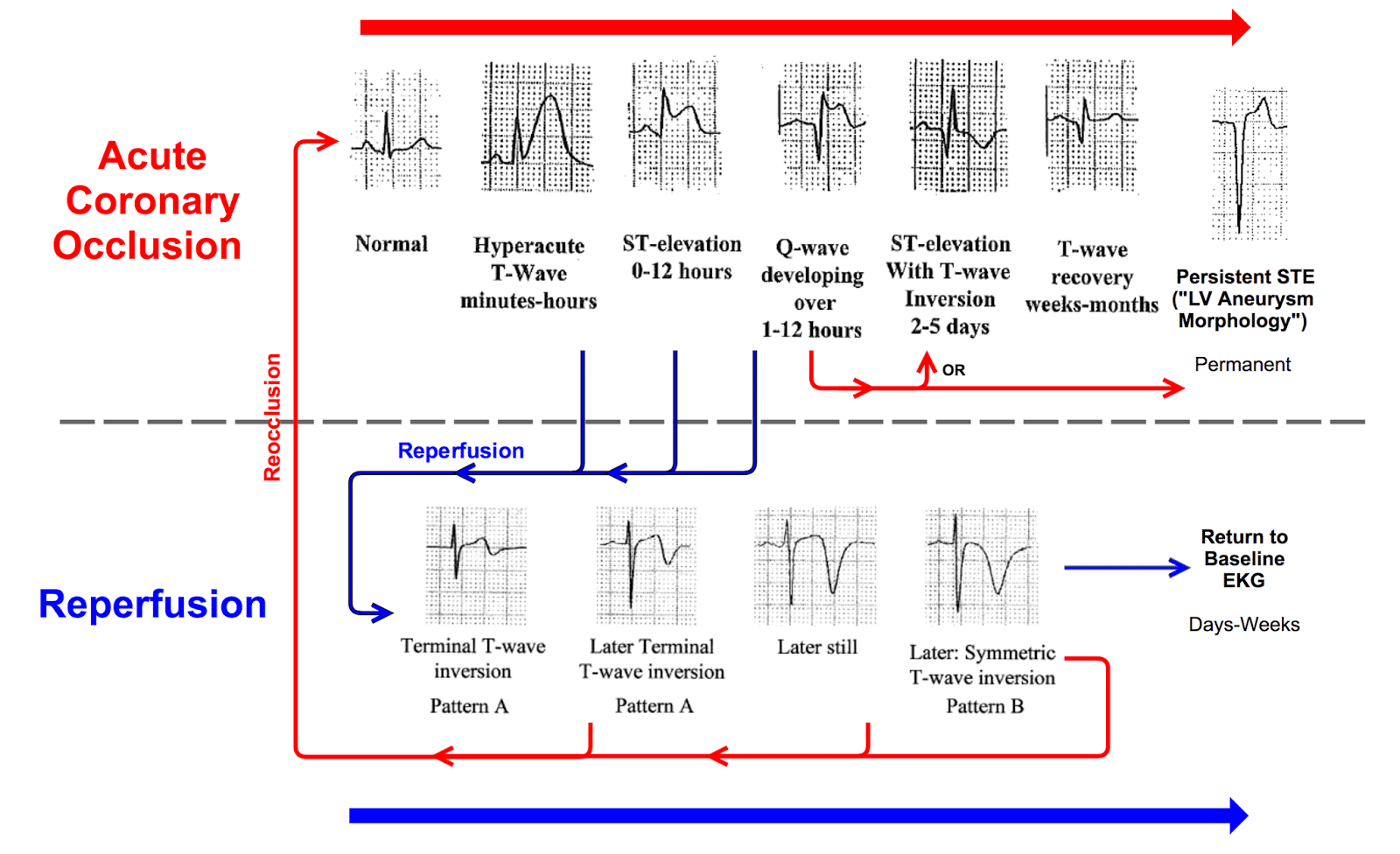
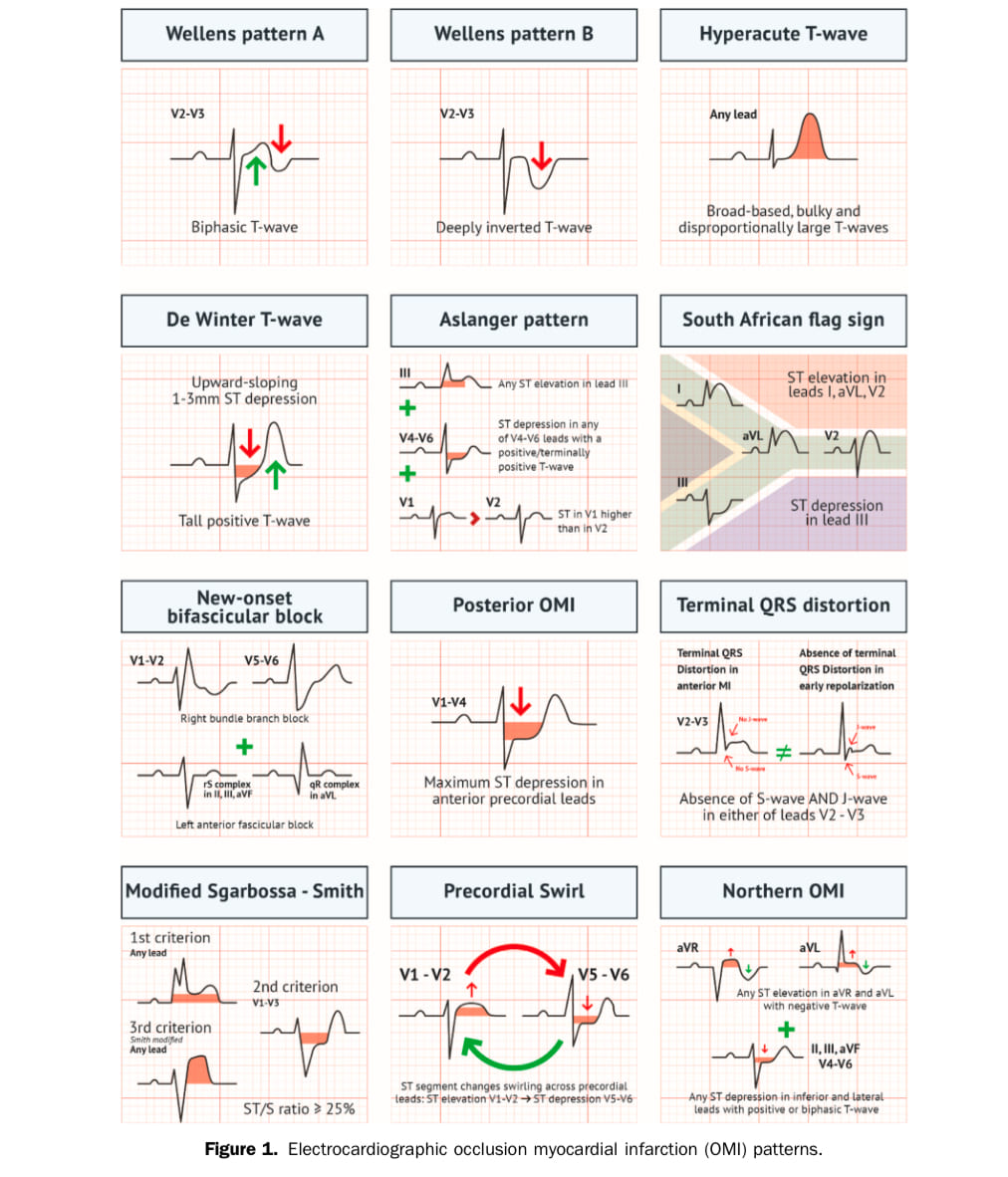
OMI ECG Patterns not meeting STEMI criteria:
- Wellens pattern A
- Wellens pattern B
- De Winter T-wave
- New-onset bifascicular block
- Modified Sgarbossa – Smith
- Aslanger pattern
- Posterior OMI
- Precordial Swirl
- Hyperacute T-wave
- South African flag sign
- Northern OMI
Mimics of OMI with ST Elevation: