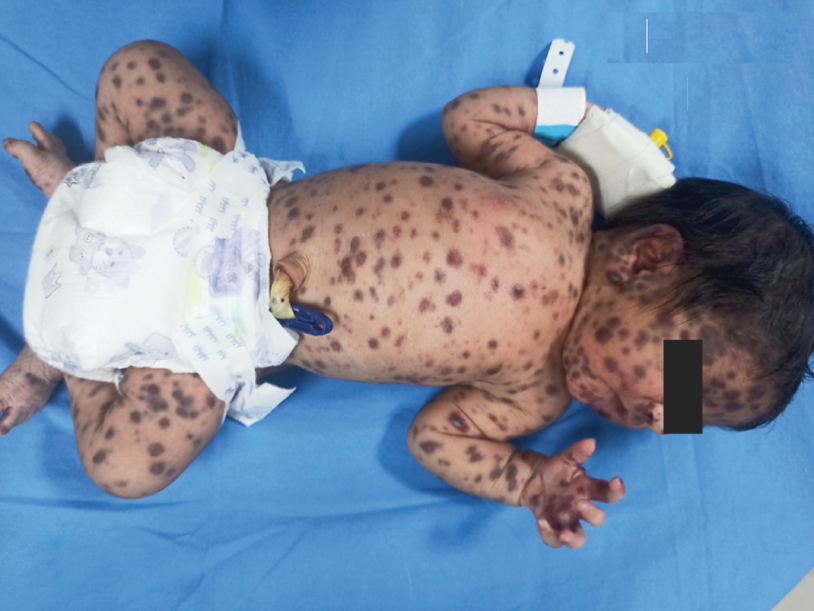
A 20-year-old male presented to an outpatient headache clinic with a 10-year history of headache, which had become daily over the past 3 months and awakened her in the middle of the night. Past history also revealed chronic musculoskeletal pain, syncope, fatigue, and hypermobility of joints.
Ehlers–Danlos syndromes (EDS) are a group of thirteen genetic connective-tissue disorders that are in the current classification, with a fourteenth type discovered in 2018.
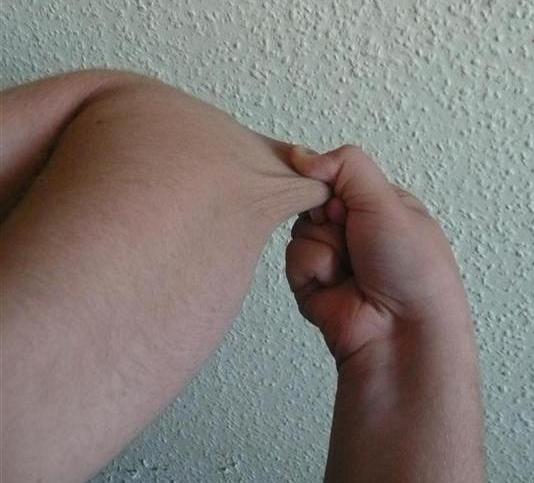
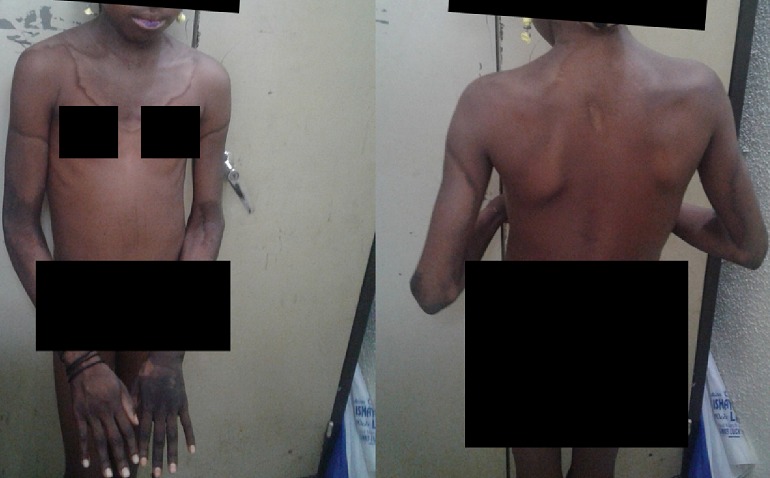
Symptoms may include loose joints, joint pain, stretchy velvety skin, and abnormal scar formation. These may be noticed at birth or in early childhood.
Complications may include aortic dissection, joint dislocations, scoliosis, chronic pain, or early osteoarthritis.
EDS occurs due to variations of more than 19 genes that are present at birth.[1] The specific gene affected determines the type of EDS. Some cases result from a new variation occurring during early development, while others are inherited in an autosomal dominant or recessive manner. Typically, these variations result in defects in the structure or processing of the protein collagen.
Diagnosis is often based on symptoms and confirmed with genetic testing or skin biopsy but people may initially be misdiagnosed with hypochondriasis, depression, or chronic fatigue syndrome.
No cure is currently known and treatment is supportive in nature. Physical therapy and bracing may help strengthen muscles and support joints. While some forms of EDS result in a normal life expectancy, those that affect blood vessels generally decrease it.