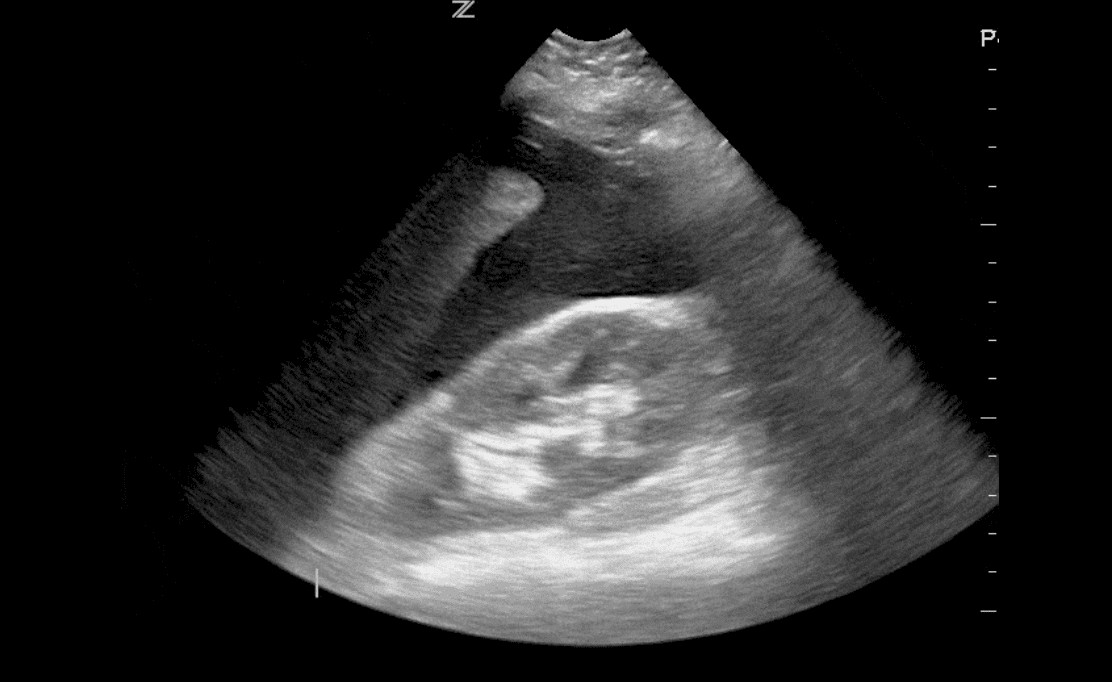
Infants with a larger thymus at 2 months of age had a significantly higher risk—over sixfold—for developing atopic dermatitis (AD) by age 2. The risk remained high—over fivefold—for early-onset AD before 6 months.
Study Overview: A prospective study of 300 full-term infants at Rigshospitalet, Copenhagen (2017–2019) tracked thymus size via ultrasound at birth, 2 months, and 12 months. AD severity was assessed using the Eczema Area and Severity Index (EASI). Out of 300, 290 infants completed the 2-year follow-up.
Results:
-
AD prevalence by age 2: 34.1%
-
High thymic index at 2 months:
-
Moderate correlation between thymic index and EASI score at 2 months (r = 0.39); no correlation at birth or 12 months.
Clinical Insight: The association held even after adjusting for filaggrin (FLG) gene mutations and family history, indicating thymic size may independently predict AD risk.
Source: Jacob P. Thyssen, MD, PhD, University of Copenhagen; study published online July 29 in Allergy.
Limitations: Findings may be limited by potential bias from AD overrepresentation, variability in ultrasound quality, and reliance on thymus size alone.
Funding & Disclosures: Supported by multiple foundations including LEO, Lundbeck, Novo Nordisk, and others. Some authors reported financial ties or employment with sponsors.